The Importance of ISO 13485 Compliance

Editor’s note: Gala explains how the international standard ISO 13485 defines the requirements for the quality management systems of medical device manufacturers. If you want your medical devices to meet international quality requirements, you are welcome to turn to ScienceSoft’s team for healthcare IT consulting.
The global market of medical devices is steadily expanding due to the aging population, the rising incidence of chronic diseases, and the growing awareness of telemedicine among the general public. Compliance with the ISO 13485 standard allows a medical device manufacturer to produce and sell safe and high-quality medical devices for various purposes.
ISO 13485: An Overview of the Latest Revision
ISO 13485 is the international quality management standard for medical devices, issued by the International Organization for Standardization (ISO). It is based on the general quality management standard ISO 9001 (more specifically, on its version ISO 9001:2008), which is industry-independent. ISO 13485 was developed and published by ISO in 1996 and has been revised several times since then. The latest revision of the standard was released in March 2016.
ISO 13485:2016 applies to medical device manufacturers who want to adjust their quality management systems (QMS) and processes in accordance with international requirements throughout all stages of a medical device life cycle. The stages include design, development, production, storage, delivery, technical support, and disposal of a medical device. Here, you can review the list of documents required for a medical device manufacturer’s QMS to comply with ISO 13485:2016.
ISO 13485:2016 Key Requirements
ISO 13485:2016 applies to organizations having various roles in a medical device production life cycle, regardless of their size and type (public or private), unless expressly indicated in the text of the standard. The requirements of the standard are also applicable to the related services provided by the organization. For example, if a company that manufactures laboratory microscopes also provides the services of their calibration and repair, the ISO 13485 requirements extend to the services as well.
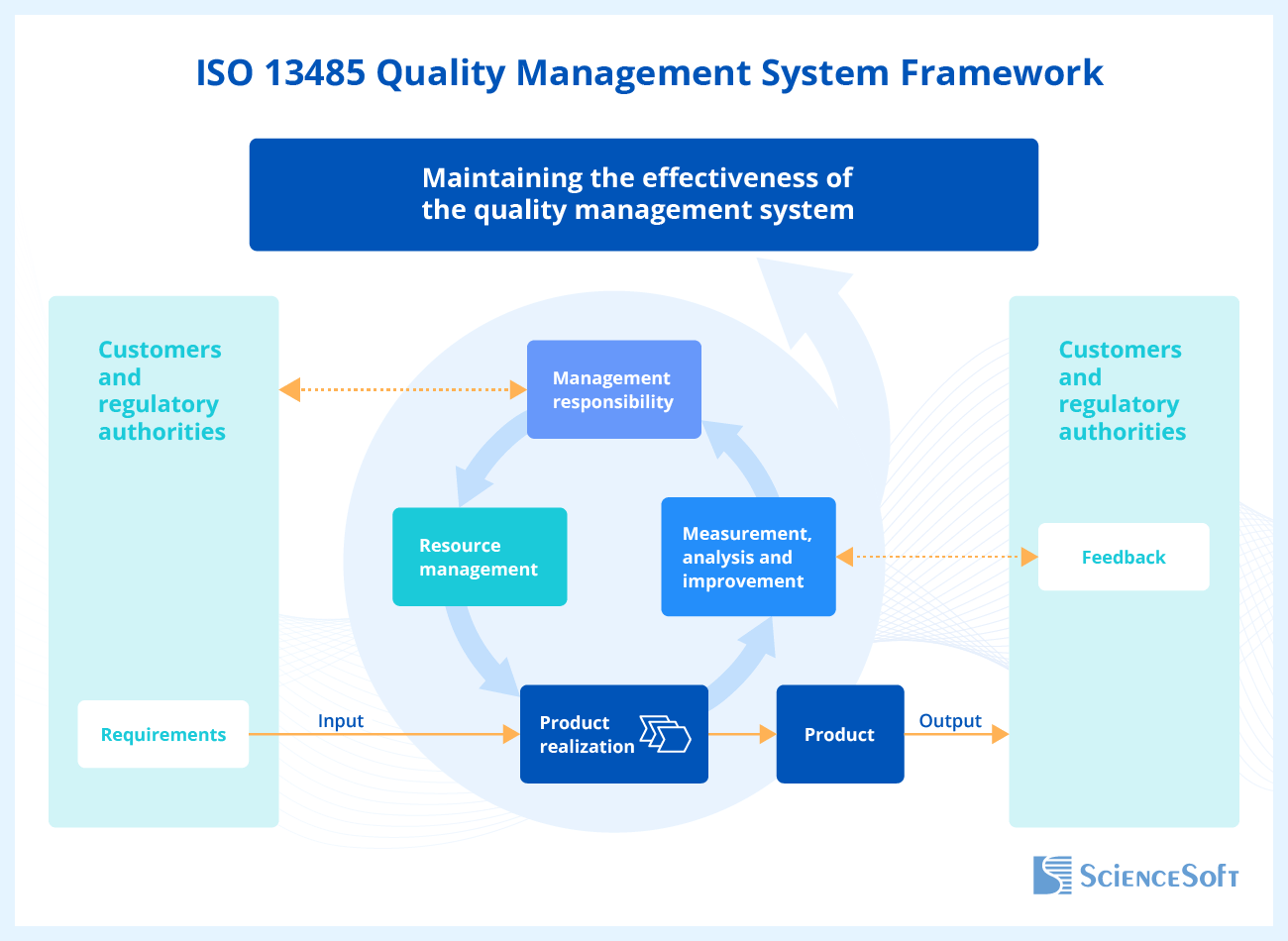
ISO 13485:2016 consists of eight parts: parts 1-3 are introductory, and parts 4-8 describe the mandatory requirements for the medical device manufacturers’ QMS. Let’s take a look at parts 4-8 in more detail.
Part 4. Quality Management System
This section of the standard sets out the general requirements for the QMS and its documentation, namely, the quality manual and the medical device file.
Part 5. Management Responsibility
According to this part of the standard, in addition to the implementation of the quality management system, the company executives must establish the quality policy and objectives and regularly review the QMS processes to identify deficiencies and fix them.
Part 6. Resource Management
The requirements of this section of the standard apply to all resources of an organization, including infrastructure, buildings, personnel, work environment, and resources needed for environmental control.
Part 7. Product Realization
According to this part of the standard, the manufacturing organization should fulfill several important conditions of product realization. First, the manufacturer needs to plan and control such stages of a medical device life cycle as design and development (including the analysis of each stage with an indication of its participants and dates), the device verification, validation, and the procedure for managing changes.
Part 8. Measurement, Analysis, and Improvement
This section of the standard stipulates measures and processes that need to be applied to the medical device production processes to ensure QMS conformity. It takes into account the level of customer satisfaction with the quality of the purchased medical device, internal audit (e.g., for checking and assessing the processes of risk management, evaluating employee satisfaction with the working conditions), etc.
The Benefits of Being ISO 13485:2016-Compliant
Although the ISO 13485 certification is voluntary, more than 33,000 companies globally have been certified as of December 31, 2023. That is because compliance with the requirements of this standard provides medical device manufacturers with a number of benefits. At the base level, the manufacturer’s intention to comply with ISO 13485 requirements demonstrates a focus on quality, customer needs, and generally improves the company’s image on the market. But more importantly, many countries base their QMS requirements on this standard or reference it directly, so compliance is often expected. This makes the official certification a valuable asset in streamlining your way to the market.
How to Achieve Compliance With ISO 13485:2016
- Introduce a quality policy that defines measurable quality objectives.
- Appoint a Management Representative to oversee QMS implementation and ongoing compliance.
- Conduct a gap analysis or equivalent assessment to determine the QMS processes required and prioritize areas for development.
- Develop an implementation plan with clear objectives, tasks, responsible persons, required documents, and timelines to cover the identified deficiencies.
- Draft, review, and approve the required QMS procedures and work instructions. Include SOPs for document control, training, risk management, design control, CAPA, etc.
- Deliver targeted training on the new or updated procedures to all relevant personnel. Track participation and comprehension with sign-offs or quizzes.
- Establish a document control policy, outlining the traceability, version control, and access control rules.
- (If your device production process involves purchased materials or services) Define and implement supplier qualification, monitoring, and re-evaluation procedures.
- Define and enforce a record retention policy. Records should be maintained for at least the lifetime of the medical device or as specified by applicable regulatory requirements (but no less than 2 years from the product’s release).
- Schedule regular internal audits and formal management reviews of the processes, introduce targeted improvements based on the findings, and update documentation accordingly.
- Establish (and document) procedures for feedback gathering, complaint handling, and post-release product efficiency monitoring.
Make Your QMS ISO 13485-Compliant
A well-established quality management system helps avoid quality problems, optimize the design and development of a medical device, and identify weaknesses in the production cycle. If you need expert assistance in establishing an effective QMS and achieving ISO 13485 compliance, feel free to contact ScienceSoft’s healthcare IT team.
Launch your Project
Medical Device Software Development by ScienceSoft
Looking to develop medical device software? Our certified BAs, developers and QA specialists work together to deliver highly secure and reliable healthcare solutions.


