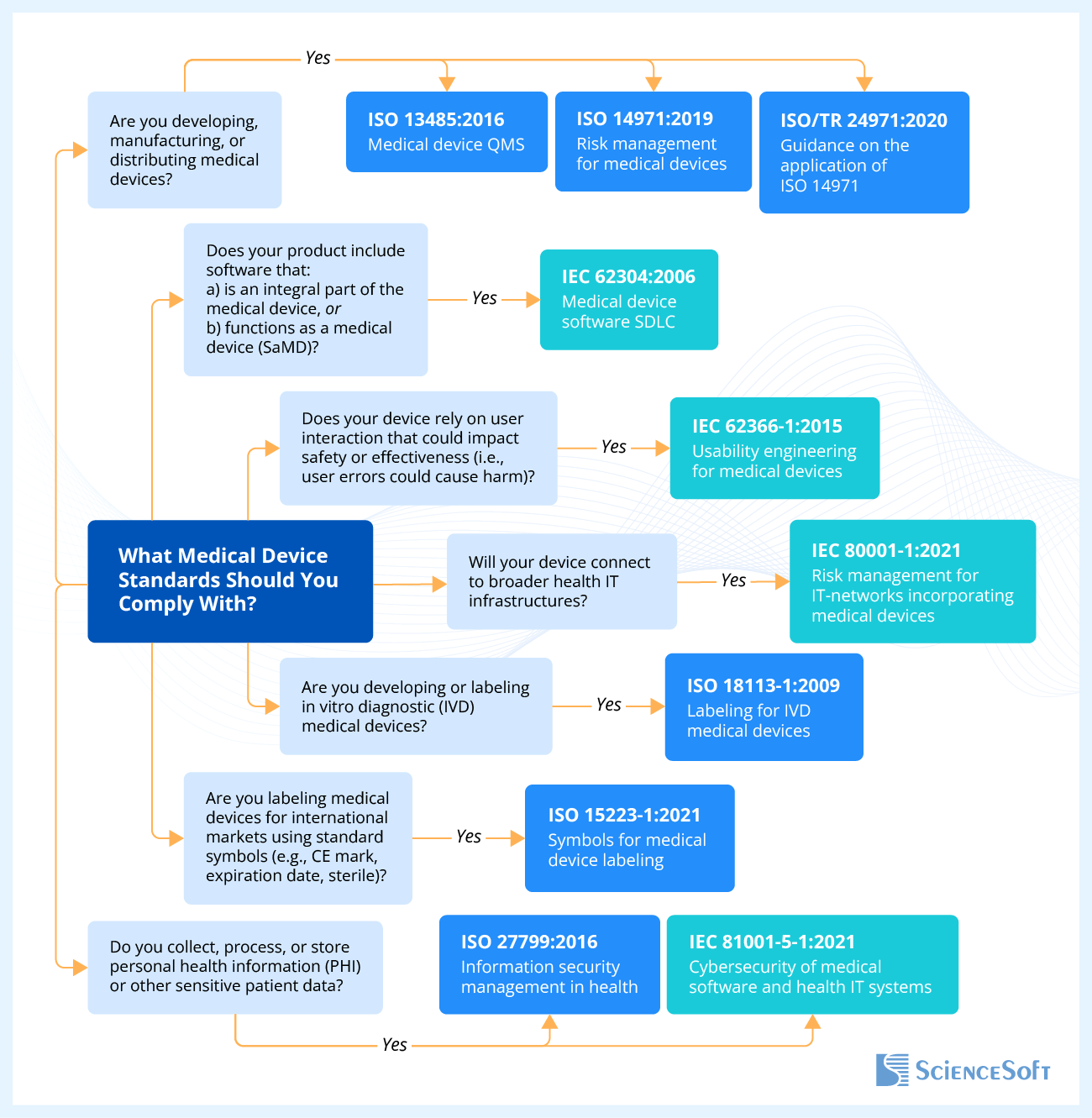
Medical Device Standards: Purpose And Popular Examples

Editor’s note: Gala explains why complying with standards for medical devices is a must for manufacturers and gives examples of the most important international medical device standards that are set by the International Organization for Standardization (ISO) and the International Electrotechnical Commission (IEC). If you are not sure how to identify applicable international medical device standards for the device you are bringing to market or planning to implement, you are welcome to turn to ScienceSoft’s team for healthcare IT consulting.
The global medical device market stood at $542.21 billion in 2024 and is projected to reach $886.68 billion by 2032. The key market drivers are the growing awareness among the general population in the field of telemedicine and the rising prevalence of chronic disorders.
The high level of consumer interest in smart medical devices, close attention of regulatory authorities, and the resulting competition in the market forced manufacturers to pay special attention to the quality of their products. With hackers targeting healthcare technology to attack hospitals or elicit confidential patients’ information, medical device cybersecurity has become a burning issue for businesses in healthcare. The safety and effectiveness of medical devices aimed at monitoring patients’ health, preventing and managing diseases are ensured by medical device standards adherence. Additionally, for medical device manufacturers, compliance with international standards means an opportunity to enter the global market. Many regional regulations (e.g., EU MDR) are built upon them, making compliance a smart long-term investment.
Who Creates International Medical Device Standards?
When talking about region-independent standards, two organizations are typically brought up:
The International Organization for Standardization (ISO), as the name suggests, is dedicated to the creation of global standards for a wide range of industries, including health IT and medical devices. ISO standards for medical devices establish safety and quality requirements for their design, production, and post-market surveillance. Their main goal is to facilitate trade and cooperation between companies from all over the world.
The International Electrotechnical Commission (IEC) publishes international standards for electrical, electronic, and related technologies, which involve certain aspects of medical device development. By creating harmonized development rules, IEC aims to minimize friction caused by regional requirement variations and increase public access to safe and high-quality medical devices.
Major International Medical Device Standards
For medical device manufacturers, compliance with international standards means an opportunity to enter the global market. Usually, the name of the standard is indicated by a combination of a few digits and several numbers. The letters at the beginning of a standard are an abbreviation for the issuing organization, digits before the colon is the number assigned to the standard by its developers, and the digits after the colon are the year of its issue.
ISO standards
ISO 13485:2016 is the main Quality Management System (QMS) standard for medical devices and software as a medical device. It is intended to ensure that medical devices are designed, manufactured, delivered, and disposed of when necessary. Although voluntary, this standard has become a benchmark for medical device manufacturers. The latest data shows that more than 33,000 companies across the world have already been officially certified, highlighting the global impact of ISO 13485 compliance.
ISO 27799:2016 stipulates requirements for medical device manufacturers to maintain the confidentiality and security of the personal health information of the patients who will use the medical device.
ISO 14971:2019 describes terminology, principles, and a process for risk management of medical devices, software as a medical device, and in vitro diagnostic medical devices.
ISO 15223-1:2016 refers to symbols that are provided for the labeling of medical devices and can also be used in the accompanying documentation for a medical device and on its packaging.
ISO 18113-1:2009 stipulates general principles and essential requirements for information supplied by the manufacturer of in vitro diagnostic medical devices.
IEC standards
IEC 62304:2006 specifies the lifecycle process for the development of medical device software.
IEC 62366-1:2015 describes the process of analyzing, specifying, developing, and evaluating medical device usability. It also describes the assessment and mitigation of risks during the correct use of the device.
IEC 80001-1:2021 establishes risk management requirements for medical devices connected within broader healthcare IT environments.
IEC 81001-5-1:2021 specifies cybersecurity requirements for the secure lifecycle of health software and health IT systems.
Make Your Medical Devices Compliant With International Standards
Compliance with standards like ISO helps medical device manufacturers prove the high level of quality and safety of their products, as well as manufacturers’ concern about the environment and consumer interests. If you would like to enter the medical device market and make your device comply with the required standards, ScienceSoft’s healthcare IT team will be glad to help.
Launch your Project
Medical Device Software Development by ScienceSoft
Looking to develop medical device software? Our certified BAs, developers and QA specialists work together to deliver highly secure and reliable healthcare solutions.



